Abstract
Introduction: Key characteristics of immune thrombocytopenia (ITP) include immune-mediated platelet destruction/impaired production, with resultant thrombocytopenia and increased bleeding risk. Durable response to current therapies remains an unmet need, particularly in the relapsed/refractory setting. Rilzabrutinib is the first oral, reversible, covalent inhibitor of Bruton tyrosine kinase designed to target immune-mediated pathways in ITP without inhibiting normal platelet aggregation. Initial phase I/II results in ITP demonstrated rapid and durable efficacy with rilzabrutinib that was well-tolerated at all dose levels, including the optimal 400 mg bid dose. Interim results on rilzabrutinib effects in patients with relapsed/refractory ITP were previously reported. Here we present long-term data from a larger group of patients who initiated rilzabrutinib at 400 mg bid and are continuing in the long-term extension (LTE) period.
Methods: This ongoing, global phase I/II study (NCT03395210) enrolled adult patients from 8 countries with relapsed or refractory ITP who previously responded to ≥1 prior ITP therapy. Eligible patients with 2 baseline platelet counts of <30×10 9/L no less than 7 days apart in the 15 days before baseline received oral rilzabrutinib at starting doses of 200 or 400 mg QD, 300 or 400 mg bid for 24 weeks; intrapatient dose escalation was permitted to improve efficacy. Stable doses of concomitant corticosteroids and thrombopoietin receptor agonists were allowed for patients with inadequate platelet response. The primary endpoints were safety and achievement of ≥2 consecutive platelet counts ≥50×10 9/L and increased ≥20×10 9/L from baseline without rescue medication. Responding patients could continue in the LTE period with rilzabrutinib at the optimal 400 mg bid dose.
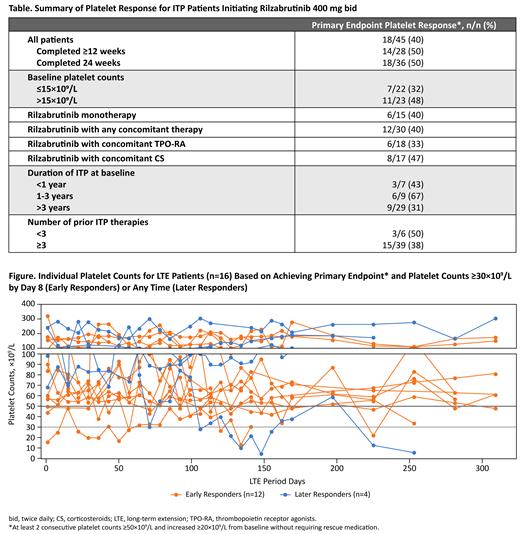
Results: As of June 1, 2021 in 60 patients, 45 patients initiated rilzabrutinib 400 mg bid and to date, 16 patients with durable, stable response proceeded to LTE at 400 mg bid. At enrollment for patients initiating rilzabrutinib 400 mg bid, median age was 49 y (range, 19-74), median duration of ITP was 6.1 y (range, 0.4-52.5), and median platelet count was 15×10 9/L (range, 2-33×10 9/L). Patients were heavily pretreated with a median of 6 prior treatment events (range, 1-53), including 24% with prior splenectomy. Overall, 18/45 patients (40%) achieved the primary endpoint. In primary responders, platelet counts of ≥30×10 9/L, ≥30×10 9/L with ≥20×10 9/L above baseline, and ≥50×10 9/L were maintained for a median of 95%, 86%, and 72% of weeks, respectively. Median time to first platelet counts of ≥30×10 9/L, ≥30×10 9/L with ≥20×10 9/L above baseline, and ≥50×10 9/L were 8.5 (7-134), 11.5 (7-134), and 12.5 (8-142) days, respectively. According to subgroup analyses, primary platelet responses were consistently >30% irrespective of baseline platelet counts, the use of concomitant therapy, duration of ITP, or number of prior therapies (Table). LTE patients received rilzabrutinib for an overall median duration of 462 days (range, 303-764). At LTE entry, patients had a median platelet count of 87×10 9/L (range, 16-321×10 9/L). In addition to all LTE patients achieving the primary endpoint during the main treatment period, these patients maintained platelet counts of ≥30×10 9/L, ≥30×10 9/L with ≥20×10 9/L above baseline, and ≥50×10 9/L, for a median of 100%, 96%, and 90%, of weeks, respectively, during the LTE period (Figure). Treatment-related treatment-emergent adverse events (TEAEs; all grade 1/2) were reported in 27/45 patients (60%); the most common were 36% diarrhea and 31% nausea, and all others were <10%. Only 3 patients had grade 1/2 treatment-related TEAEs during the LTE period, supporting a favorable safety profile with longer treatment. There were two related grade 1 bleeding events (conjunctival hemorrhage and contusion), two related grade 2 infections that resolved on treatment (erysipelas during the main treatment period and upper respiratory tract infection during the LTE), but no related thrombotic events or deaths.
Conclusion: Oral rilzabrutinib 400 mg bid was well-tolerated and had durable, clinically significant platelet responses across subgroups and with extended treatment in patients with heavily pretreated ITP. Continued study in the ongoing, randomized phase III LUNA3 trial (NCT04562766) will further assess the magnitude and durability of rilzabrutinib's clinical benefit in ITP.
Kuter: Actelion (Syntimmune), Agios, Alnylam, Amgen, Argenx, Bristol Myers Squibb (BMS), Immunovant, Kezar, Principia, Protalex, Rigel, Takeda (Bioverativ), UCB: Research Funding; Up-to-Date: Patents & Royalties: Up-To-Date; Rubius: Current equity holder in publicly-traded company; Actelion (Syntimmune), Agios, Alnylam, Amgen, Argenx, BioCryst, Bristol Myers Squibb (BMS), Caremark, CRICO, Daiichi Sankyo, Dova, Genzyme, Immunovant, Incyte, Kyowa-Kirin, Merck Sharp Dohme, Momenta, Novartis, Pfizer, Principia, Protalex, Protalix, Rigel: Consultancy, Other: grant support and consulting fees; Platelet Disorder Support Association: Membership on an entity's Board of Directors or advisory committees. Mayer: Principia: Research Funding. Jansen: 3SBIO, Novartis: Other: Travel, accomodations, expenses; Advisory Board Novartis: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Other: Travel, Accommodations, Expenses. McDonald: Bayer, Sobi, Novartis, Amgen, argenx: Honoraria; Grifols: Research Funding. Baker: Roche, Janssen-Celeg: Membership on an entity's Board of Directors or advisory committees; Bayer, Takeda, Pfizer, Daiichi Sankyo, CSL Behring, Roche, Amgen, Celgene, Rigel Pharmaceuticals, Abbvie, Sanofi, MorphoSys AG, Acerta Pharma, Jansen-Cileg, Bristol-Myers Squibb, Boehringer Ingelheim, Portola, Technoclone, Alexion: Research Funding. Bird: Novartis, Amgen: Speakers Bureau. Garg: Amgen Janssen Novartis Sanofi Takeda: Honoraria; Takeda Janssen Novartis Sanofi: Other: Travel Accommodations, Expenses; University Hospital Leicester: Current Employment. Gernsheimer: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: personal fees ; Amgen: Honoraria; Cellphire: Consultancy; Dova: Consultancy, Honoraria; Principia: Membership on an entity's Board of Directors or advisory committees, Research Funding; Rigel: Membership on an entity's Board of Directors or advisory committees, Research Funding; NHLBI: Research Funding. Ghanima: Amgen, Novartis, Pfizer, Principia Biopharma Inc- a Sanofi Company, Sanofi, SOBI, Griffols, UCB, Argenx: Consultancy; Amgen, Novartis, Pfizer, Bristol Myers Squibb, SOBI, Griffols, Sanofi: Honoraria; Bayer, BMS/Pfizer: Research Funding. Bandman: Sanofi: Ended employment in the past 24 months. Arora: Principia Biopharma Inc, a Sanofi Company: Ended employment in the past 24 months. Burns: Sanofi: Ended employment in the past 24 months. Yao: Sanofi: Current Employment. Daak: Sanofi: Current Employment. Sourdille: Sanofi: Current Employment. Thomas: Chinook and Equillium Biopharma: Current holder of individual stocks in a privately-held company; Chinook: Membership on an entity's Board of Directors or advisory committees; Equillium Biopharma: Current Employment; Principia, a Sanofi Company: Ended employment in the past 24 months; Equillium Biopharma: Current equity holder in publicly-traded company. Neale: Principia Biopharma/Sanofi: Ended employment in the past 24 months; Principia Biopharma: Divested equity in a private or publicly-traded company in the past 24 months. Cooper: Sanofi and Principia: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel, accommodations expenses; Principia and Sanofi: Consultancy.
Rilzabrutinib is an investigational therapy being evaluated in a clinical study for the treatment of patients with immune thrombocytopenia.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal